Master Thesis in Medical Science
with a major in biomedicine
Effects
of interleukin-10 on the MHC class I-restricted antigen presentation under
normal and hypoxic conditions
by
Mikael Altun*#
* Department of Pathology, Harvard Medical School, 200 Longwood Avenue, Boston, Massachusetts 02115, USA
# Microbiology and Tumor Biology Center, Karolinska Institutet, S-171 77 Stockholm, Sweden
2001
Supervisors:
Prof. Hidde L. Ploegh*, PhD
Benedikt M. Kessler*, PhD
Maria Teresa Bejarano#, MD, PhD
Table
of Contents
1.1
General role of Interleukin-10
1.2
Role of IL-10 in tumor biology
1.3
Effects of IL-10 on MHC class II antigen presentation
1.4
Effects of IL-10 on MHC class I antigen presentation
1.4.1
Cytosolic proteases involved in antigen presentation
2.2
Antibodies, reagents, and solutions
2.3
Preparation of cell lysates
2.4
Preparation of radiolabeled active site-directed probes with [125I]
2.5
Labeling of Proteasome with active site-directed probes
2.9
[35S]-metabolic labeling and immunoprecipitation experiments
3.1
MHC class I maturation in the presence of IL-10
3.2
Effects of IL-10 on proteasomal activity
3.3
Influence of INF-g on the composition
of the proteasome
3.4
Examination of proteasome and non-proteasomal activity under hypoxic conditions
Conclusions
and future perspectives
The
cytokine IL-10 is secreted by a variety of hematopoietic cells and has
pleiotropic stimulatory and suppressive activities on the immune system. In
addition, IL-10 exerts anti-angiogenic effects, which may expose tumor cells to
hypoxic conditions. We studied the effects of IL-10 on the MHC class I antigen
presentation pathway under normal or hypoxic conditions and in the presence of
INF-g. As a system, we utilized a Burkitt lymphoma
cell line, DG75, which was transfected with either a human or a viral form of
IL-10. The presence of IL-10 affects neither the turnover nor the maturation of
the MHC class I molecule, and does not seem to have an effect on the activity
of the cytosolic 26S proteasome and AAF-AMC hydrolyzing protease activity under
normal conditions. Under hypoxic conditions, no change in 26S proteasome
activity, but an increase in AAF-AMC hydrolyzing activity was observed in cells
expressing the human form of IL-10. We noted an increase in lower molecular
weight polypeptides, providing evidence for the involvement of lower molecular
weight proteases, and excluding larger proteases such as tripeptidyl-peptidase
II. All cell lines contained the constitutively expressed proteasome, and
immunoproteasome expression could be induced by exposure to INF-g.
However, IL-10 transfected cells expressed a different ratio between
constitutive and inducible immunoproteasome subunits. This suggests that IL-10
has a negative effect on the formation of the immunoproteasome, representing
the first evidence for a cytokine-dependent negative regulation of
immunoproteasome formation. Thus, immune responses may be modulated by IL-10,
by altering cytosolic proteolysis and therefore the antigen pool presented by
MHC class I molecules.
Abbreviations
Adamantane-acetyl-tyrosinyl-triaminohexanoyl-
trileucinyl-vinylsulphone AdaYAhx3L3VS
Ala-Ala-Phe-Tyr-7-amino-4-metyl-coumarine AAF-AMC
Antibody-dependent cell-mediated cytotoxity ADCC
Antigen Ag
Antigen
presenting cell APC
Bleomycin
hydrolase BH
Boc-Leu-Arg-Arg-Tyr-7-amino-4-metyl-coumarine Boc-LRR-AMC
Burkitt lymphoma BL
Cathepsin Cat
Cobaltchloride CoCl2
Cysteine Cys
Cytotoxic T-lymphocyte CTL
Dendritic cell DC
Epstein-Barr Virus EBV
Endoplasmic Reticulum ER
Fast performance liquid chromatography FPLC
Fetal calf serum FCS
Hypoxia inducible factor HIF
Immunoglobulin Ig
Interferon INF
Interleukin IL
Lymphoblastoid cell line LCL
Major histocompatibility complex MHC
Methionine Met
Natural killer NK
Nitric oxide synthase NOS
Phosphate-buffered saline PBS
Platelet derived growth factor PDGF
Polyvinylidene fluoride PVDF
Puromycin-sensitive aminopeptidase PSA
Sodium dodecyl sulfate SDS
Succinyl-Gly-Gly-Leu-Tyr-7-amino-4-metyl-coumarine Suc-GGL-AMC
Succinyl-Leu-Leu-Val-Tyr-7-amino-4-metyl-coumarine Suc-LLVY-AMC
Transporter associated with antigen presentation TAP
Trichlorolacetic acid TCA
Tripeptidyl peptidase II TPP II
Tumor necrosis factor TNF
Ubiquitin Ub
Vascular endothelial growth factor VEGF
1.
Introduction
1.1 General role of Interleukin-10
Interleukin-10 (IL-10) is a 18 kDa homodimeric cytokine (Vieira et al., 1991), which is secreted by activated T cells (Yssel et al., 1992), B cells (Matthes et al., 1993), monocytes (de Waal Malefyt et al., 1991), and binds to specific receptors expressed on all haematopoietic cells (Liu et al., 1994). It is a cofactor for the growth and differentiation of B cells (Defrance et al., 1992; Rousset et al., 1992) and mast cells (Thompson-Snipes et al., 1991), and also has strong immunosuppressive and anti-inflammatory activities (Moore et al., 1993). This cytokine has a variety of different immune modulating functions. It reduces antigen-specific T-cell proliferation by diminishing the stimulatory function capacity of antigen presenting cells (APC’s), such as macrophages/monocytes and dendritic cells (DCs) (Morel et al., 1997), via downregulation of major histocompatibility complex (MHC) class II expression (Koppelman et al., 1997). It has also been suggested that IL-10 influences the size of the macrophage population. Exposure of IL-10 to macrophages may lead to differentiation or apoptosis. During an immune response, the presence of IL-10 and immunoglobulin G (IgG) may contribute to an accumulation of macrophages at an inflammatory site, especially macrophages that express Fc receptor FcgRIII (CD16). The Fc receptor FcgRIII is also expressed on Natural Killer (NK) cells that recognize the IgG1 and IgG3 subclasses, followed by cytolysis, a so called antibody-dependent cell-mediated cytotoxicity (ADCC). IL-10 can induce CD16+ macrophages with an ADCC effector cell potential at the expense of macrophages with antigen-presenting and costimulatory capacity (Wang et al., 2001). Due to its negative regulatory effects on IL-12 and interferon (INF)-g production (D'Andrea et al., 1993; Seder et al., 1993), IL-10, together with IL-4, directs the immune response more towards Th2 immunity rather than Th1 (Moore et al., 1993; Mosmann, 1991; Mosmann and Moore, 1991).
The Th1 and Th2 immune responses are related to the subset of CD4+ T-cells that are expanded depending on the cytokine environment. The exact mechanism of CD4 T cell differentiation is not fully understood, but in vitro experiments have shown that CD4 T cells that are stimulated in the presence of IL-12 and INF-g develop into Th1 cells and inhibit Th2 differentiation. Th1 cells secrete INF-g, the main macrophage activating cytokine, and lymphotoxin (LT-a and tumor necrosis factor-b), which also activates macrophages and inhibit B-cells activation. CD4 T cells activated in the presence of IL-4 and IL-6 tend to differentiate to Th2 cells, as IL-4 and IL-6 promote the differentiation of Th2 cells, whereas IL-4 and IL-10 inhibit the generation of Th1 cells. Th2 activated cells secrete IL-4 and IL-5, which activate B-cells, and IL-10, which deactivates macrophage activation (Arai et al., 1990).
1.2 Role of IL-10 in tumor biology
In addition to its role in modulating the immune system, IL-10 is also involved in tumor biology. IL-10 can either promote or suppress tumor growth based on the analysis of different tumor models. This may be explained by the amount of IL-10 secreted by the tumor as well as the pleiotropic effects of IL-10. Epstein-Barr virus (EBV) transformed Lymphoblastoid cell lines (LCLs) are non-tumorigenic in SCID-mice. In contrast, Burkitt lymphoma (BL) cells, which are EBV-negative, are highly malignant in SCID mice. There are several differences between the cell-lines, one being LCLs express a viral form of IL-10 encoded by the EBV, while BLs usually do not express at all. BLs have a chromosomal translocation that results in constitutive activation of the oncogene, c-myc, which LCLs do not carry (Klein, 1983). It has been shown that BLs transfected with genes coding human or viral IL-10 have a remarkably decreased tumorigenicity and angiogenic activity in SCID mice (Cervenak et al., 2000). The human IL-10 protein (hIL-10) has 84% amino acid identity to a form of IL-10 produced by the EBV virus (vIL-10). vIL-10 lacks the immunostimulatory capacity and has a 1000-fold lesser affinity for the IL-10 receptor. When Isoleucine in position 87 on hIL-10 is replaced with the corresponding amino acid in the vIL-10 protein, alanine, the hIL-10 loses its immunostimulatory capacity, and the affinity for the receptor decreases a 100-fold. It seems that one amino acid determines the immunostimulatory activity of IL-10 (Ding et al., 2000).
IL-10 seems to have anti-angiogenic properties (Cervenak et al., 2000), for which the exact underlying mechanisms are not fully understood. The generation of factors that regulate the production of angiogenic molecules is controlled by proteolysis. For example, the hypoxia inducible factors (HIFs) are key factors in the response to oxygen availability, and are regulated by oxygen-dependent proteolysis. HIFs regulate the expression of several angiogenic molecules including vascular endothelial growth factor (VEGF), nitric oxide synthase (NOS), platelet derived growth factor (PDGF) and others (Carmeliet and Jain, 2000).
|
Table 1 Mechanisms used by tumors to escape from immunosurveillance (Salazar-Onfray, 1999) |
|
· Failure of MHC expression, avoiding the MHC-restricted immune-mediated anti-tumor response. · Heterogeneity of tumor antigen expression. · Failure of the expression of adhesion or accessory molecules by tumor cells and/or host dendritic cells required for efficient T cell interaction. · Secretion of immunosuppressive substances by the tumor. · Induction of immune non-responsiveness via anergy induction or clonal deletion of tumor-reactive T cell. · Induction of suppressor cells or cells secreting inhibitory cytokines by the host immune system. · Tumor-induced changes in T-cell signal transduction molecules. · Utilization of stimulatory factors produced by the activated immune system for tumor cell growth. |
1.3 Effects of IL-10 on MHC class II antigen
presentation
The responses of MHC class II restricted antigen-specific T-cells are inhibited by IL-10 when human monocytes are used as antigen presenting cells (APC). This is correlated with the downregulation of MHC class II molecules on the surface of the monocytes. IL-10 may not affect MHC class II transcription, polypeptide synthesis, subunit assembly, or antigentic peptide loading. The newly synthesized mature MHC class II are localized to the MHC class II compartments, but are prevented from reaching the plasma membrane by IL-10 and the accumulation of internalized MHC class II complexes in intracellular vesicles occurs. This indicates that IL-10 affects antigen presentation by regulating MHC exocytosis and recycling (Koppelman et al., 1997).
In DCs, IL-10 alters the upregulation of cathepsin S (Cat S) and cathepsin B (Cat B) activities and levels of enzyme expression. Suppression of Cat B and Cat S activities results in delays of MHC class II sodium dodecyl sulfate stable dimer formation and impairs antigen (Ag) degradation (Fiebiger et al., 2001).
1.4 Effects of IL-10 on MHC class I antigen
presentation
It has been shown that IL-10 reduces target cell recognition by cytotoxic T-lymphocytes (CTL) (Matsuda et al., 1994; Salazar-Onfray et al., 1995) by downregulating the expression of MHC class I molecules (Matsuda et al., 1994). Some of these effects are related to a general inhibition of the Th-1 immune response, attributed to an MHC class I antigen downregulation and inhibition to INF-g production. However, IL-10 induces the Natural Killer (NK) cell activity that leads to increased tumor rejection (Moore et al., 1993; Salazar-Onfray et al., 1997; Salazar-Onfray et al., 1995). IL-10 mediated downregulation of MHC class I may provide an explanation for the observed NK-cell mediated tumor rejection, which is in agreement with the “missing self hypothesis” that have been postulated (Ljunggren and Karre, 1990). It was proposed that the downregulation of MHC class I molecular expression may be influenced by the NFkB transcription factor in the short-term and by the transporter associated with antigen presentation 1+2 (TAP1+2) molecules in long-term exposure to IL-10 (Terrazzano et al., 2000)
1.4.1 Cytosolic proteases involved in antigen
presentation
Intracellular proteases generate antigens for presentation by MHC class I molecules. One of the most studied and well-characterized example is the 26S proteasome, a multicatalytic protease complex (Baumeister et al., 1998). In addition to generating antigens for MHC class I, the proteasome also is linked to the ubiquitin (Ub) linked pathway, which is responsible for degradation of most cytosolic proteins. The proteasome is involved in many biological processes such as cell cycle control by degradation of cyclins, cell differentiation by destruction of transcription factors or metabolic enzymes, removal of misfolded or improperly assembled proteins, and stress responses through processing or degrading transcriptional regulators (Coux et al., 1996; Deveraux et al., 1994; Voges et al., 1999).
The 26S proteasome consists of a 700 kDa cylindric 20S
proteolytic core and two 900 kDa 19S cap complexes. The 20S proteasome contains
28 subunits, which are divided into a-
and b-subunits and are arranged in a
cylindrical shape. The cylinder is itself divided into four heptamer rings,
where the two central rings are made up of b-subunits
(b1-b7),
capped at both ends by a ring of a-subunits
(a1-a7)
(Fenteany et al., 1995;
Groll et al., 1997; Lowe et al., 1995). The b-subunits harbor the catalytic active parts of the proteasome,
where three of the seven subunits are catalytically active per ring. The proteasome cleaves proteins into
peptides of 7-10 amino acid of average length (Kisselev et al., 1999). The catalytically active
subunits may cooperate with each other in their role of cleaving peptides, but
each catalytic subunit may execute a preferred cleavage. The preferred peptide
bond cleavages proceed hydrophobic (chymotrypsin-like activity), basic
(trypsin-like activity), and acidic (peptidylglutamyl-like activity) residues,
but the specificity of cleavage in each case is rather broad (Bogyo et al., 1998; Kessler et al., 2001;
Kisselev et al., 1999; Schmidtke et al., 1998).
The 19S regulatory complex, which is responsible for the
recognition of Ub-proteins, deubiquitination, unfolding, and processing
substrates to the 20S proteolytic core can be divided into a base- and lid-
subcomplex. The 19S complex uses the multi-ubiquitin signal to destroy
intracellular proteins targeted for destruction. The base is composed of six
ATPases of the triple “AAA” family (ATP associated with variety of cellular
activities) and two non-ATPase subunits, which bind to the 20S core. It is the
ATPase subunits that are believed to be involved in the unfolding of substrates
and channeling them into the proteases. The residual eight subunits of the 19S
form the lid, and its function is not yet fully understood (Braun et al., 1999; Glickman et al., 1999;
Strickland et al., 2000).
In the presence of INF-g,
the 26S proteasome changes its composition, which affects several of the 20S
proteasome subunits and the 19S regulatory complex. The three catalytically
active subunits in the 20S proteasome, Y (b1),
Z (b2) and X (b5) are replaced by three INF-g
inducible subunits (immunosubunits), LMP2 (b1i),
MECL1 (b2i) and LMP7 (b5i), which also are catalytically active.
This newly formed 20S proteasome is more commonly referred to as the
“immunoproteasome” (Griffin et al., 1998;
Groettrup et al., 1997; Schmidt and Kloetzel, 1997). With these new subunits, the
cleavage pattern of the proteasome is altered. Several studies addressing the
generation of antigenic epitopes from immunoproteasomes suggest that this
system has evolved to optimize antigentic peptide production with MHC class I
specificity, therefore enhancing presentation by MHC molecules and subsequent
immune responses (Sijts et al., 2000). But the role of INF-g is more complicated than that. It has been
evidenced that this cytokine not only induces the formation of “pure”
immunoproteasome, but also that of 20S complexes in which immunosubunits and
constitutive subunits are simultaneously incorporated. For example, b5i is found to be present in the 20S without
the other immunoproteasome subunits (Frentzel et al., 1994). These observations raise the
question about the general role of the immunoproteasome and its “hybrid”
species. The 19S regulatory complex can be replaced by another complex called
the PA28 or 11S regulatory complex, which replaces one or both of the 19S
regulatory caps (Dubiel et al., 1992; Ma et al., 1992). This complex is composed of two distinct a- and b-subunits
that form a hexa or heptameric ring (Knowlton et al., 1997). The 11S complex also carries
ATPase activities, such as the 19S, but there is not so much known about the
exact function and role the 11S regulatory particles (Preckel et al., 1999).
The proteasome has a vital role in the maintenance of cell
viability. It is difficult to imagine normal cell function or survival without
a functional proteasome. Proteasome function can be inhibited pharmacologically
by non-covalent or covalent modification of proteasomal b-subunits. EL-4 lymphoma cells treated with proteasomal
inhibitor, such as NIPL3VS, die initially; however after 2-3 weeks,
a small population recovers and starts growing, referred to as “adapted cells”.
These cells have a dramatic reduction of the proteasomal function, but manage
to survive by compensating for the loss of proteasome function using an
additional proteolytic system. One of these alternative proteases that gets
upregulated is tripeptidyl-peptidase II (TPP II) (Glas et al., 1998; Wang et al., 2000). TPP II has a rod-shaped structure,
slightly larger than the 20S proteasome, and degrades polypeptides by exo- as
well as trypsin-like endoproteolytic cleavage (Balow et al., 1986;
Geier et al., 1999).
Also, when the oncogen c-myc is overexpressed, intracellular proteolysis is
shifted to alternative pathways, where proteases such as TPP II are upregulated
(Gavioli et al., 2001). This may lead to alternative
peptide cleavage due to changes in specificities, which will give rise to
different peptides to be presented by MHC class I molecules. Tumor cells with
these properties have an easier tumor escape and become more tumorgenic and
aggressive. Other proteases have been suggested to contribute to the generation
of peptides for the MHC class I, such as Bleomycin hydrolase (BH) and puromycin-sensitive
aminopeptidase (PSA). These two peptidases are not well characterized as much
as the previously discussed proteases, but they seem to have a role acting
downstream of the proteasome in the antigen processing pathway by trimming
peptides to the right size for the MHC class I molecule (Stoltze et al., 2000).
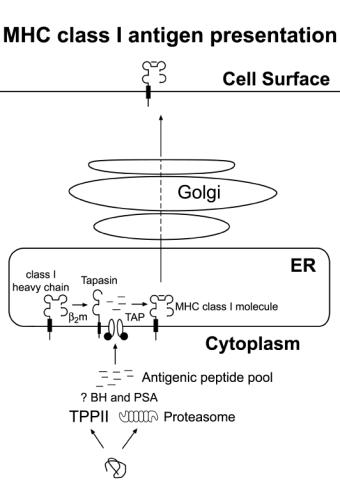
Figure
1.
Schematic illustration of the MHC class I antigen presentation pathway.
Proteins in the cytosol are degraded by proteases such as the 20S proteasome or
tripeptidyl peptidase II (TPP II). Peptides that are generated can be further
trimmed by other protease such as Bleomycin hydrolase (BH) and
puromycin-sensitive aminopeptidase (PSA) before they are transported into the
endoplasmic reticulum (ER) by TAP transporter (transporter associated with
antigen presentation), which delivers a peptide that binds to the MHC class I
molecule and completes its folding and release. The MHC class I molecule goes
through the Golgi apparatus, where it gets glycosylated, before it goes up to
the cell surface to be exposed to the immune system.
Aim of the project
In the present project, we aim to study the effect of IL-10 on the MHC class I antigen presentation pathway. As a biological system, we used the Burkitt lymohoma cell line (DG75) that is transfected with a vector expressing either human IL-10 (hIL-10) or viral IL-10 (vIL-10). The maturation of MHC class I will be addressed by metabolic labeling and pulse-chase experiments. Proteasomal activity in different cell lines will be characterized by using the active site-directed probe AdaYAhx3L3VS (Kessler et al., 2001). Western blot analysis will be used to determine the composition of the constitutive and/or immunoproteasomes. Both tryptic-like and chymotryptic-like activities of the proteasomes present in these cell lines will be examined using fluorescent substrates that are specific for the activity. As a readout for non-proteasomal protolysis, AAF-AMC hydrolyzing activity will be measured in cell extracts to test for any differences in other protease activities, such as TPP II, present in the different cells, and if IL-10 has a role in up- or downregulating them. The cells will be treated with INF-g, which plays an important role in the induction of immunoproteasome formation. The question is whether IL-10 may interfere with the formation of immunoproteasome induced by INF-g.
Proteolysis is essential in the control of angiogenesis and may influence the physiological process in which endothelial cells divide and migrate to form new vessels. Most angiogenic and antiangiogenic molecules are products derived from the proteolytic cleavage of existing cellular factors. Examining if IL-10 has any effect on angiogenesis, the cells will be subjected to hypoxic conditions, and proteasome activity and AFF-AMC hydrolyzing activities will be measured with fluorogenic peptid-substrate assays. The proteases complex will be separated by size using gelfiltration to test if any changes that may occur can be detected in cytosolic proteolysis under hypoxic conditions, with or without the presence of IL-10.
2.
Material and Methods
2.1 Cell lines
The Burkitt lymphoma like B-cell line DG75 used in this project was isolated from a primary abdominal lymphoma, with a chromosomal translocation (8;14). These cells are EBV negative (Ben-Bassat et al., 1977). The cells were transfected with a vector containing genes coding viral IL-10 (vIL-10), human IL-10 (hIL-10), or an empty vector (a kind gift from Maria Teresa Bejarano; Cervenak et al, 2000). As control cells, EL-4 cells and HeLa cells were used. The EL-4 cells express both constitutive and immunoproteasomes, while HeLa only expresses the constitutive proteasome. The DG75 wild type and the EL-4 cell line were cultured in RPMI 1640 (Gibco; Rockville, MD, USA), supplemented with 2mM glutamine, 100 U/ml penicillin, 100 U/ml streptomycin, and 10% heat-inactivated fetal calf serum (FCS). All of the transfected cells were cultured in RPMI 1640 with HEPES (Gibco; Rockville, MD, USA), supplemented with 2mM glutamine, 100 U/ml penicillin, 100 U/ml streptomycin, 10% heat-inactivated FCS, and 750 mg/mL of the selection marker G-418. HeLa cells were cultured in DME (Gibco; Rockville, MD, USA) supplemented with 2mM glutamine, 100 U/ml penicillin, 100 U/ml streptomycin, and 10% heat-inactivated FCS. All cells were cultured at 37°C/5% CO2.
2.2 Antibodies, reagents, and solutions
Primary antibodies used in this project were the monoclonal rabbit anti-b1i (1:1500; Affinity, UK) and rabbit anti-b5i (dilution used for western blot analysis 1:1500; Affinity, UK) against the immunosubunits, and rabbit anti-b1 (1:1500; Affinity, UK) against the constitutive proteasomal subunit. As secondary antibodies, goat anti-rabbit coupled with streptavidine-horseradish peroxidase were used at a dilution of 1:5000 (Southern Biotechnology, Birmingham, AL, USA) with phosphate-buffered saline (PBS) containing 0.5% tween.
Radiolabeling of the active
site directed probe AdaYAhx3L3VS
(Adamantane-acetyl-tyrosinyl-triaminohexanoyl-trileucinyl-vinylsulphone; (Kessler et al., 2001)) was performed with Iodo-genŇ
(Pierce, Rockford, Il, USA) and radioactive [125I] (Amersham
Pharmacia Biotech, Piscataway, NJ, USA). Cobaltchloride
(CoCl2, Acros Organics, USA) was used in hypoxia treatment of
cell lines. Radioactive [35S]-methionine (Amersham Pharmacia
Biotech, Piscataway, NJ, USA) was used in metabolic labeling experiments. The
fluorescent substrates Succinyl-Leu-Leu-Val-Tyr-7-amino-4-metyl-coumarine
(Suc-LLVY-AMC, Sigma, St Louis, MO, USA),
Boc-Leu-Arg-Arg-Tyr-7-amino-4-metyl-coumarine (Boc-LRR-AMC, Sigma, St Louis,
MO, USA), Succinyl-Gly-Gly-Leu-Tyr-7-amino-4-metyl-coumarine (Suc-GGL-AMC,
Sigma, St Louis, MO, USA) and Ala-Ala-Phe-Tyr-7-amino-4-metyl-coumarine
(AAF-AMC, Sigma, St Louis, MO, USA) are used for fluorescent substrate specific
assays. Interferon-gamma (INF-g, Roche,
Indianapolis, IN, USA) treatment of cell lines was performed with recombinant
human INF-g.
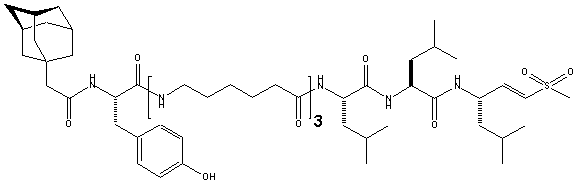
Figure
2. Chemical
structure of active site-directed probe AdaYAhx3L3VS.
A commassie blue R-250 stock solution (24 gram commassie brilliant blue (Bio-Rad, Hercules, CA, USA), 600ml methanol, 120ml acetic acid) was used for protein staining. DMSO/PPO solution (220 gram PPO (2,5-diphenyloxazole) dissolved in 800ml DMSO) was used to measure increase in detection of [35S]-methionine radiolabeled proteins. 3x reducing Sodium dodecyl sulfate (SDS) sample buffer (3ml Glycerol, 1.5ml betamercaptoethanol (2.12M), 0.9 gram SDS, Upper tris (4x) 3.75, H20 up to 10ml, and bromphenolblue), Lower 4x SDS-page gel buffer pH 8.8 (90.85 gram tris Base, 4.8ml SDS 25% (or 2 gram), H20 up to 500ml), upper 4x SDS-PAGE gel buffer pH 6.8 (30.3 gram tris Base, 4.8ml SDS 25% (or 2 gram), H20 up to 500ml), and 10x Laemmli running buffer (1440 gram Glycine, 100 gram SDS, 300 gram Tris base) were used to run SDS-PAGE gels. Western blot transfer buffer (WBT; 300ml 10x WBT (100mM Tris base, 1.0M glycine, H2O up to 1 liter), 600ml Methanol (20% final conc.) and 2.1 liter of H2O) was used to transfer proteins to polyvinylidene fluoride (PVDF; Millipore Corp., Bedford MA, USA) membrane.
2.3 Preparation of cell lysates
Cells were harvested and spun down for five minutes at 1,000xg and the supernatant was removed. The cells were washed by resuspending in cold phosphate-buffered saline (PBS) and spun down for five minutes at 1,000xg three times. The pellet was resuspended and transferred to a 1.5 ml eppendorf tube to be spun down for 10 minutes at 3,800xg and the supernatant removed. The cells were kept at –80°C if not further processed immediately. An equal volume of glass beads (10 microns; Sigma, St Louise, MO, USA) as thawed cells and twice the volume of homogenization buffer pH 7.4 (50mM Tris-base, 5mM MgCl2, 250mM sucrose, and fresh 2mM ATP and 1mM DTT) to cell pellet were added to ensure the correct conditions for preserving proteasomal activity. The cells were vortexed at 4°C for 10 min and spun down at 17,900xg at 4°C for 10 minutes. The supernatants were transferred to a new 1.5ml eppendorf and the pellets were resuspended in half of the amount of homogenization buffer and vortexed for another 10 minutes, spun down and the resulting supernatant was pooled with the first supernatant. The supernatants were spun down at 17,900xg for 25 minutes at 4°C, and the supernatant was transferred to ultracentrifuge tubes and spun 72,200xg for 1 hour at 4°C in the ultracentrifuge (Beckman, Fullerton, CA, USA). To determine the protein concentration, Bradford assay at a 1:5 dilution with water (BIO-RAD, Hercules, California), was used and samples were measured in a UV-detector (Beckman, Fullerton, CA, USA) at 592nm and used Bovine serum albumin (BSA) as standards.
To enrich for proteasome, differential centrifugation was applied. The supernatants from the 72,200xg were further spun in the ultracentrifuge (Beckman, Fullerton, CA, USA) at 72,200xg at 4°C for 5 hours. The pellet was resuspended in the homogenization buffer and the protein concentration was measured as described above.
For the preparation of lysates containing active cathepsins, the cells were detergent lysed. 200ml of the lysis buffer pH 5.0 (Na acetate 50mM, 0,1% Triton X-100) was added. The cells were vortexed for 15 minutes and then incubated on ice for 30 minutes. The samples were spun for 25 minutes at 17,900xg at 4°C. The protein concentration of the supernatant was measured as described above.
2.4 Preparation of radiolabeled active site-directed
probes with [125I]
50mg of the compounds, AdaYAhx3L3VS and JPM 565, were resuspended in 30ml of Acetonitrile and transferred to 1.5ml eppendorf tubes coated with 100mg of Iodogen. 10ml of 50mM phosphate buffer pH 7.4 was added. One mCurie of [125I] was added and the samples vortexed for 30 seconds. For purification a Sepack C18 Waters column was used. The column was washed with 10ml of Acetonitride and then 10ml of 50mM phosphate buffer pH 7.4. The compound was added to the column and washed with 20ml of phosphate buffer to remove the unbound compound and iodine. The compound was eluted with Acetonitrile and collected in three one-ml fractions. The activity was determined, by measuring 3ml of the fractions using a g-radiation counter (Beckman, Fullerton, CA, USA). The radiolabeled compound was stored in a ledbox at -80°C and used within a month.
2.5 Labeling of Proteasome with active site-directed
probes
To label the proteasome, 25mg of the pH 7.4 lysates were prepared in 60ml of pH 7.4 homogenizing buffer. The radiolabeled active site-directed probe AdaY[125I]Ahx3L3VS was added (5x105 cpm) and the samples were incubated for two hours at 37°C. After the incubation, 30ml of 3x SDS sample buffer was added and the samples were incubated for 3 minutes at 95°C to denature the proteasome. The tubes were spun down for one minute at 17,900xg and 45ml of the lysates were analyzed on a 12.5% SDS-PAGE gel. After migration, the gel was disassembled and stained with commassie-blue staining solution for 30 minutes (15ml commassie stock, 250ml methanol, 200ml water and 50ml acetic acid) for protein staining. To distain the gel, it was incubated for 2x30 minutes in a shaker at room temperature with 250ml of water, methanol and acetic acid (250ml water, 200ml methanol, 50ml acetic acid). The gel was then washed with 1:1 water and methanol for 2x30 minutes in a shaker at room temperature and finally incubated with water, methanol and glycerol (231ml water, 30ml methanol and 9ml glycerol) for 30 minutes in shaker at room temperature. The gel was then put on a wetted filterpaper and covered with cellophane. The gel was put in a gel-dryer (Bio-Rad, Hercules, CA, USA) and the air-bubbles were eliminated before the vacuum was applied. The gel was dried for two hours at 80°C. The gel was dry, it was put in a cassette (Fisher Scientific, Pittsburgh, PA, USA) on a X-OMAT AR film (Kodak, Rochester, NY, USA) and exposed at -80°C for one to three days. The film was then processed in a developer (Alpha Tek, USA) and the bands corresponding to proteasomal b subunits were quantified using a Hirschman densitometer (Alpha Innotech Corp, UK).
To label cathepsins, 15mg of the pH 5.0 lysates were diluted with pH 5.0 detergent lysis buffer up to 60ml. The radiolabeled JPM 565 compound (5x105 cpm) was added and samples were incubated for two hours at 37°C. After incubation, 30ml 3x SDS reducing sample buffer was added and samples were boiled at 95°C for 3 minutes to denature the proteins. 45ml of the samples were analyzed by 12.5% SDS-PAGE gel and autoradiography.
2.6 Western blotting
25mg of the lysates were put in homogenization buffer up to 60ml and 30ml of 3x SDS-sample buffer was added and samples were boiled for 3 minutes at 95°C to denature the proteasome. The tubes were spun down for one minute at 17,900xg and 45ml of the lysate was separated on a 12.5% SDS-PAGE gel. A PVDF membrane (hydrophilic) was reduced to a size that covered the gel. The membrane was incubated in methanol for five minutes and in the transfer buffer for five minutes to equilibrate. The gel was put in the sandwich cassette (sponge, 3 filter papers, membrane, gel, 3 filter papers, and sponge, all wetted in transfer buffer). The sandwich cassette was closed and put in the transfer apparatus (Bio-Rad, Hercules, CA, USA) and run at 250mA for 3 hours at 4°C. After the transfer the membrane was put in PBS containing 0.5% tween 20 and 5% milk to block unspecific binding. It was transferred on a shaker for 1-2 hours at room temperature. The membrane was then washed three times in PBS containing 0.5% tween 20. The primary monoclonal antibodies, rabbit anti-b1i (1:1500), rabbit anti-b5i (1:1500), and rabbit anti-b1 (1:1500) were diluted in 20ml of PBS containing 0.5% tween per membrane and incubated on a shaker for 1 hour at room temperature. The membrane was washed three times for 10 minutes in PBS containing 0.5% tween 20. The secondary antibody goat anti-rabbit coupled with streptavidine-horseradish peroxidase (1:5000) was added and samples were incubated for one hour at room temperature. The membrane was then washed three times for 10 minutes with PBS containing 0.5% tween 20 followed by two washings of five minutes duration in PBS and finally one five minute wash in water. An ECL solution mix (NEN-Dupont, Boston, MA, USA) was added to the membrane for one minute and removed. The membrane was then exposed to a X-OMAT AR film and processed in a developer in order to visualize the bands.
2.7 Enzymatic assay
5mg of the enriched pH 7.4 lysates enriched with proteasome (5h pellets) were put in homogenization buffer containing fluorescent substrates, suc-GGL-AMC and suc-LLVY-AMC for chymotryptic-like activity, Boc-LRR-AMC for tryptic-like activity and AAF-AMC for AAF-hydrolyzing activity. The samples were read in a fluorofor plate reader (Cytoplate, Perseptive Biosystems, Framingham, MA, USA) at 0 minutes and every 15 minutes for the first hour and then every 30 minutes for the next three hours using an excitation/emission ratio of 380/460 nm. The results are shown as bar graphs where the wild type was set as 100% at the 90-minute time point.
2.8 Cell treatments
2.8.1 INF-g treatment
To determine the best conditions for INF-g treatment, three x 106 cells of DG75 vector were treated with 0, 20, 100, 500, and 3000 international U/ml of INF-g and incubated for 48 and 72 hours at 37°C/5% CO2. As a control, two x 106 HeLa cells were used and treated under the same conditions. The cells were harvested and lysed as previously described. The cell-lines were labeled with the active site labeling probe, AdaY[125I]Ahx3L3VS, and run on a 12.5% SDS-PAGE gel and exposed on a X-OMAT AR film overnight.
Ten million cells per cell-line were treated with 100
international U/ml of INF-g for 48
hours at 37°C/5%
CO2. The cells were harvested and split into two groups, one for
studying the proteasome and the other for studying the cathepsins. The cells
were spun down and washed (see above) and kept at -80°C until used. The cells
were lysed and labeled as described above for proteasome and cathepsin lysing
and labeling. The samples were separated on 12.5% SDS-PAGE and analyzed as
described in the previous section of labeling
on proteasome with active site-directed probes.
2.8.2 Cobaltcloride treatment
Three x 106 cells of the all the cell-lines were incubated with or without 75mM of CoCl2 for 24 and 48 hours at 37°C/5% CO2 (Jiang et al., 1997). The cells were harvested and lysed as described above. The protein concentration was determined using the Bradford assay and the chymotryptic- (with suc-GGL-AMC) and AAF hydrolyzing activity (with AFF-AMC) was determined by the enzymatic assay described above. The data was normalized to the zero time point and shown as changes through time by the mean of normalized fluorescent units.
To determine the
size of proteases that showed an induction in AAF-AMC hydrolyzing activity,
250x106 vIL-10 cl.17 cells 0.5x106/ml were treated or
untreated with 75mM CoCl2
as above. After determination of the protein concentration of the lysates, an
equal amount of each lysate was injected in a Fast performance liquid
chromatography (FPLC, Pharmacia Biotech, Piscataway, NJ, USA) and separated on a Superose 6 column
(Pharmacia Biotech, Piscataway, NJ, USA). The column was equilibrated with homogenization buffer (the total
running volume used was 120ml) at a flow-rate of 0.5ml/min. 65 fractions were
collected for each sample, and every 2nd fraction starting from
fraction 10 was analyzed for protease activity. AAF-AMC and suc-GGL-AMC were
used, and the fluorescence was measured every 30 minutes for 4 hours, as
described above. The results from the 240 minute time-point are shown in
bar-graphs, where treated and untreated cells are compared.
2.9 [35S]-metabolic labeling and
immunoprecipitation experiments
3x106 cells per lane were used, collected in 50ml Falcon tubes, centrifuged at 360xg for five minutes, after which the medium was removed. The pellet was resuspended in the medium without Methionine (Met) and Cystine (Cys) at a cell concentration of 2x107 and incubated for 45 minutes at 37°C. The cells were centrifuged at 360xg, the medium was removed and 35S-Met/Cys (NEN-Dupont, Boston, USA) at 200mCi/ml i.e. of ExpressMix in fresh medium without Met and Cys was added and the cells incubated for 15 minutes at 37°C. The cells were centrifuged, the medium was removed and the cells resuspended in normal medium (1-5x106 cells/ml) and incubated at 37°C for 0, 30, 60, and 90 minutes. After each time point, an aliquot was removed and centrifuged at 3,800xg for 5 minutes. The cell pellet was washed twice with cold PBS before lysis.
The cells were detergent-lysed using NP-40 lysis buffer (50mM Tris HCl pH 7.4, 0.5 % NP-40, 150mM NaCl, and 20mM MgCl2) with fresh 1mM PMSF and 1mM Leupeptin and samples were incubated for 30 minutes on ice. The lysates were centrifuged at 17,900xg at 4°C for 10 minutes and the supernatant was transferred into new tubes. To normalize the incorporated radioactivity, trichloralacetic acid (TCA) precipitation was used; 10ml/sample was added on Watman filterpaper and air-dried. 5% TCA was added and the paper was incubated at room temperature for 10 minutes and then rinsed with 70% ethanol, 100% acetone and air dried. Each sample was put in a scintillation tube and the radioactivity was counted in a scintillator counter (Beckman, Fullerton, CA, USA). For immunoprecipitation, the samples were normalized to the amount of TCA counts. To avoid as much background as possible, the samples were precleared by adding 3ml normal mouse serum/normal rabbit serum and incubated for 60 minutes at 4°C on a shaker followed by the addition of 50ml Staph.A (stock solution of Staph.A (Rehm et al., 2001) aliquots were washed 3 times with lysis buffer before use) and incubation for another 30 minutes at 4°C on a shaker. The tubes were centrifuged and the supernatants were transferred into new tubes and 3ml of W6/32 (Parham et al., 1979) or HC70 (Tortorella et al., 1998) immune serum was added and the samples incubated for 60 minutes at 4°C on a shaker followed by the addition of 50ml of Staph.A and incubated for an additional 30 minutes. The lysates were centrifuged and the pellet was washed four times with 1xNet buffer pH 7.4 (50mM Tris HCl, 0.5 % NP-40, 150mM NaCl, 5mM EDTA). After the last wash and centrifugation, 50ml 2xSDS sample buffer was added to HC70 treated cells and incubated for 5 minutes at 95°C, centrifuged and the supernatant transferred to new tubes to be analyzed on a 12.5% SDS-PAGE gel. Half of the W6/32 treated cells were Endo H treated (followed manufactures instructions; New England Biolabs, Beverly, MA, USA); 45ml of 1x denaturing buffer was added to the Staph.A pellet, boiled at 95°C for 5 minutes, and then cooled down to room temperature. 5ml of 10x G5 buffer was added and centrifuged at 17,900xg for one minute. The samples were split in half; to every second sample, 1.25ml of Endo H enzyme was added. The samples were incubated for 60 minutes at 37°C, centrifuged and loaded on a 12.5% SDS-page gel to be analyzed. The gels were put in PPO for 20 minutes to then be incubated in DMSO/PPO solution for 60 minutes at room temperature. The gels were washed several times and dried, and exposed to X-OMAT AR films.
3.
Results
3.1 MHC class I maturation in the presence of IL-10
The maturation of the MHC class I molecules are important for presenting peptides on the cell surface. To examine the maturation of MHC class I molecules, DG75 cells were analyzed by a pulse-chase experiment. The cells were incubated in media lacking the amino acids methionine and cysteine, followed by incubation with 35S labeled methionine and cysteine for the incorporation into newly synthesized proteins (pulse). The pulse was terminated by addition of complete medium. Cells were removed and lysed at different time-points (chase) to follow the MHC class I maturation. Properly folded MHC class I molecules were recovered by using monoclonal antibodies against properly folded MHC class I molecules, from whole lysates (W6/32). The immature heavy chains were recovered from lysate using a polyclonal-anti HC serum. Half of the samples were either treated or not with the enzyme Endo H, which is an enzyme that removes glycoproteins from ER-resident, but not mature MHC class I molecules found in the Golgi. The samples were analyzed by SDS-PAGE followed by autoradiography. We observed that IL-10 had no significant influence on the maturation process of MHC class I molecules (Figure 3). The class I molecules mature normally and the maturation rate is similar. In addition, the unfolded heavy chains disappear at the same rate, which is approximately 45 minutes (Figure 3 A and B).
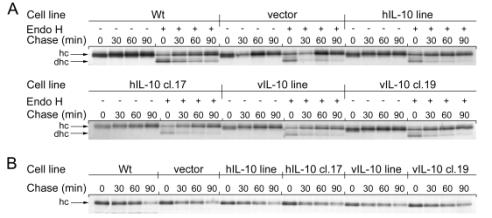
Figure
3. IL-10
does not affect MHC class I maturation or turnover. Cells were pulsed 15 min
with [35S]-labeled Met/Cys. Aliquots were taken after 0, 30, 60 and
90 minutes of chase. (A) Cells were immunoprecipitated with W6/32 and half of
the cells were treated with Endo H (+) prior to analysis by 12.5% SDS-PAGE that
separates the heavy chain (hc) and the deglycosylated heavy chain (dhc). (B)
Cells were immunoprecipitated with a polyclonal antibody recognizing unfolded
heavy chain, and analyzed by 12.5% SDS-PAGE.
3.2 Effects of IL-10 on proteasomal activity
We next tested whether IL-10 can influence the peptide pool presented by MHC class I molecules. To this end, possible effects of IL-10 on intracellular proteolysis were analyzed. The most well studied protease contributing to the generation of peptides that bind to the MHC class I, is the proteasome. One way to measure proteasome function is to assess proteasomal subunit composition and activity by using an active site-directed probe, followed by visualization of the subunits by separation on a SDS-PAGE gel and autoradiography. The compound AdaYAhx3L3VS was used, which has been shown to label all catalytic subunits of the proteasome, the constitutive and immunoproteasome. All the DG75 cell lines expressed the constitutive proteasome and not the immunoproteasome (fig.4A). To confirm this, western blot analysis was performed using antibodies against the b subunits b1i and b5i, as well as a subunit that is expressed in constitutive proteasomes, the b1 subunit. As shown in Figure 4B, there is no immunoproteasome expressed in all tested cell lines.
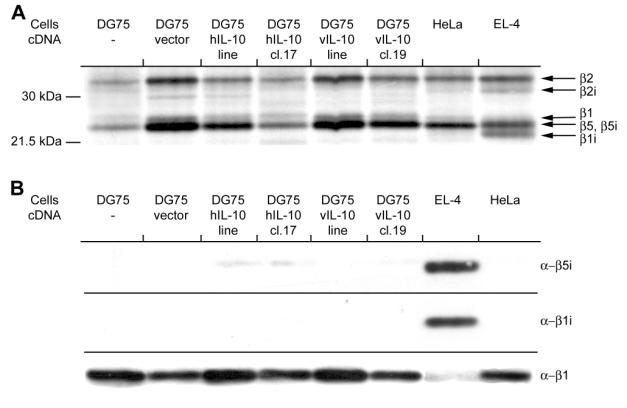
Figure 4. Constitutive proteasomes and not immunoproteasomes are expressed in DG75s cell with and without the presence of IL-10. (A) Cell lysates were labeled with the active site-directed probe Ada[125I]YAhx3L3VS, the catalytic b-subunits separated on a 12.5% SDS-PAGE and analyzed by autoradiography. (B) Westernblot analysis using antibodies against the immunoproteasome subunits b5i and b1i, and against the regular proteasome subunit b1.
Another method of visualizing proteasomal activity is by using flourogenic substrates. For the chymotryptic like activity suc-LLVY-AMC and suc-GGL-AMC, and for tryptic-like activity, Boc-LRR-AMC was used. The activities of other proteases such as TPP II, was examined using the non-proteasomal substrate, AAF-AMC. These assays showed that there is no significant difference between the proteasome activities or AAF-hydrolyzing activity detected in the different DG75 cell lines expressing IL-10 and those that do not (Figure 5).
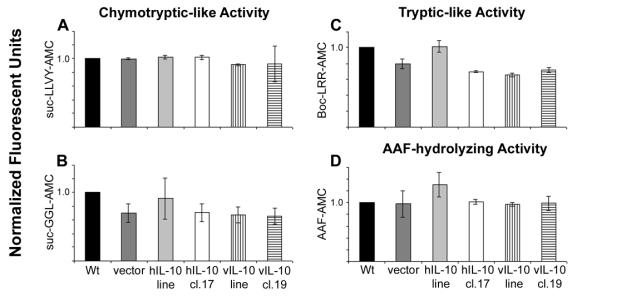
Figure 5. IL-10 does not affect intracellular proteolysis. All samples were performed in triplicate and all were normalized to wild type (wt) and are shown as means ± SEM. Chymotryptic-like activity, (A) and (B) and tryptic-like activity of the proteasome, (C) were measured. Non-proteasomal proteolysis was assessed by measuring AAF-hydrolyzing activity, (D). Activity of the fluorescent substrates suc-LLVY-AMC (A), suc-GGL-AMC (B), Boc-LRR-AMC (C) and AAF-AMC (D) were measured.
3.3 Influence of INF-g on the
composition of the proteasome
In the presence of the cytokine INF-g, the proteasome changes composition of the catalytic b-subunits from constitutive b1, b2 and b5 subunits to the immunosubunits b1i, b2i, and b5i. This gives rise to the production of a different pool of peptides for MHC class I presentation. We next examined if the DG75 cells responded to INF-g treatment and if IL-10 has any interference with the formation of immunoproteasome. The different DG75 cells were incubated with 100 U/ml of INF-g for 48 hours followed by visualization of the active b-subunits using the active site-directed probe AdaYAhx3L3VS. All the cell lines responded to INF-g stimulation, and showed a change in their subunit composition (Figure 6A), but it appeared that the IL-10 expressing cell lines contained a different ratio between constitutive- and immunoproteasome expression. The IL-10 expressing cells had less immunoproteasome expressed than the non IL-10 expressing cell lines (Figure 6B).
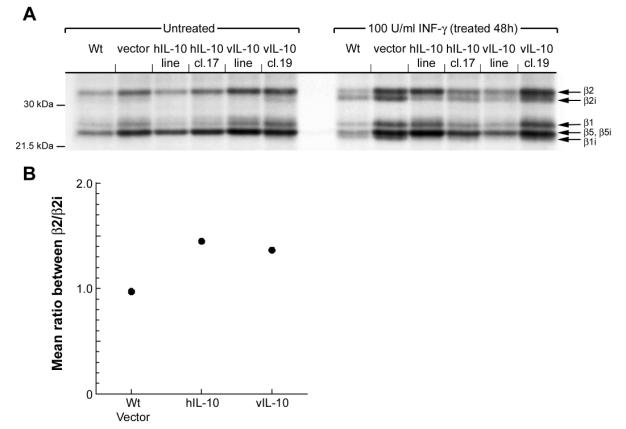
Figure
6.
Immunoproteasome formation promoted by INF-g is attenuated by IL-10. (A) Cells treated
with 100U/ml INF-g for 48h were lysed and labeled with the active site-directed probe
Ada[125I]YAhx3L3VS, catalytic b-subunits were separated on
12.5% SDS-PAGE and analyzed by autoradiography. (B) Mean ratios between the
catalytic b-subunits b2 and b2i of 20S proteasome in non-IL-10 expressing cells, hIL-10 expressing
cells and vIL-10 expressing cells were measured after quantification of the
corresponding bands.
3.4 Examination of proteasome and non-proteasomal activity under hypoxic
conditions
IL-10 has anti-angiogenic properties, which can lead to hypoxia in tumors. The turnover of many factors controlling cellular stress, such as hypoxia, is controlled by the proteasome. We therefore reasoned that hypoxia may affect the proteasome and other proteases activity, and whether IL-10 may play a role in this process. To study this, the cells were incubated with CoCl2 for 24 and 48 hours, which is a compound that induces hypoxia (Jiang et al., 1997), and lysed in order to assay the activity of the proteasome and other proteases with the aid of fluorescent substrates. Hypoxia did not have any influence on the activity of proteasomes in any of the cell lines examined, but there were some effects observed in the hIL-10 expressing cell lines (Figure 7).
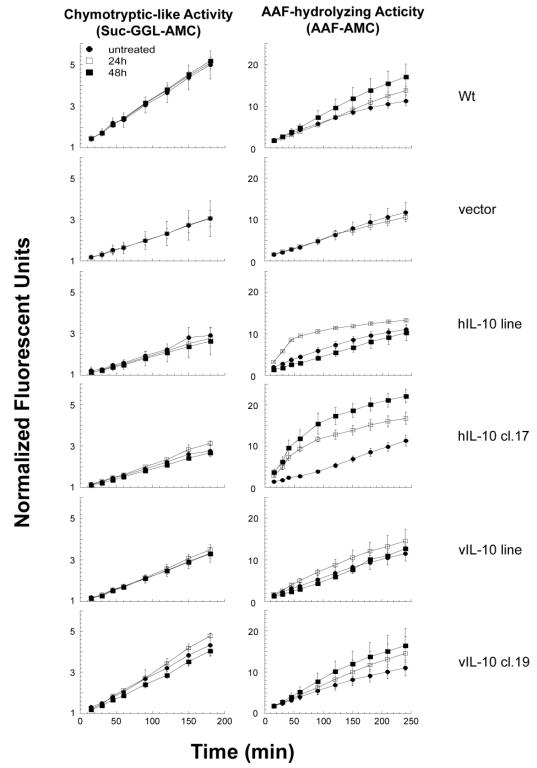
Figure 7. Hypoxia does not affect the activity of proteasomes in the presence of IL-10, but AAF-hydrolyzing activity is increased in hIL-10 expressing cells. Cells were incubated with or without 75mM of CoCl2 for 24 and 48 hours and lysates were prepared and assayed for enzymatic activities. All assays were performed in triplicate and normalized to the 0 time-point and shown as mean fluorescent units ± SEM changing over time. (A) Chymotryptic-like activity was measured with suc-GGL-AMC. (B) AAF-hydrolyzing activity was measured with AAF-AMC.
The AAF-hydrolyzing activity, indicative of non-proteasomal proteolysis, was significantly increased in cells expressing hIL-10 after CoCl2 treatment. To study if the changes in the AAF-hydrolyzing activity were due to high or low molecular peptidases, the hIL-10 cl.17 was grown with or without CoCl2 and the lysates were separated by size-exclusion column. The fractions were then analyzed using a fluorescent substrate-specific assays. The changes in AAF hydrolyzing activity is not observed in the region where high molecular polypeptides are expressed, eliminating the possibility that TPPII is involved in this process (Figure 8B). In the low molecular polypeptide region of the size-exclusion column, we observed a shift in polypeptide composition in untreated versus CoCl2 treated cell extracts (Figure 8A), indicating significant changes on the protein level under hypoxia conditions.
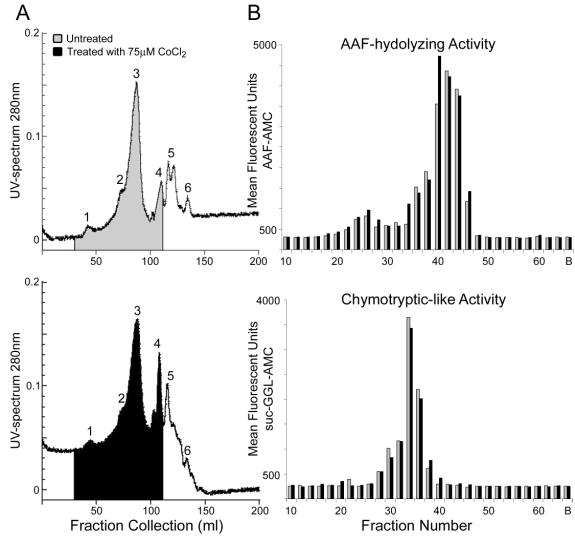
Figure 8. Changes in AAF-hydrolyzing activity in hypoxia treated cells do not affect peptidases of higher molecular weight, excluding the induction of tripeptidyl peptidase II (TPP II). Cells treated with 75mM CoCl2 were separated by size using gelfiltration and fractions of 1.5 ml were collected and analyzed enzymatically. (A) UV-spectrum at 280 nm, the upper panel shows untreated cells (grey), and the lower panel shows treated cells (black) and fractions used for enzymatic analysis are marked. (B) Fractions 10-64 were analyzed in triplicate using the fluorescent substrates AAF-AMC (AAF-hydrolyzing activity; upper panel) and suc-GGL-AMC (Chymotyptic-like activity; lower panel) and the mean of treated (black) versus untreated (grey) samples are shown per fraction.
4.
Discussion
In this work, we assessed whether IL-10 affects the MHC class I antigen presentation pathway. We observed no significant changes in MHC class I maturation or turnover, and no effects of IL-10 on the proteasome and other AAF-hydrolyzing proteases under normal conditions could be detected. However, IL-10 seems to have a negative effect on immunoproteasome formation in the presence of INF-g. Under hypoxic conditions, we observed a slight increase in AAF-hydrolyzing activity in cells expressing the human, but not the viral form of IL-10.
The roles of cytokines in tumor biology have been well studied. A tumor cell can be considered as a pathogen the same way infectious microbes are to the host. The task of the immune system is to recognize and subsequently destroy cells infected with virus or bacteria. Tumor cells are also eliminated in a set of reactions. Cytokines are important players of the immune system for determining what type of immune response is going to be activated, such as Th1 or Th2 response (Arai et al., 1990). There are different strategies to escape the immune system, which are exploited differently by microbial pathogens or tumors. One escape mechanism is based on downregulating or modifying the MHC class I pathway to make the tumor invisible to the immune system (Table 1). Modulating the amount of MHC class I molecules on the cell surface, or modifying the pool of peptides presented by the MHC class I molecule, can be alternative ways the tumor can evade the immune system.
MHC class I maturation and turnover seems to be unaffected by the presence of IL-10. The smaller differences observed are non IL-10 dependent, and seem to be more specific to the individual cell lines. This is in agreement with the MHC class I expression expressed on the cell surface, which has been previously reported for these cell lines (Cervenak et al., 2000). This is analogous to MHC class II presentation, where the effects of IL-10 are focused on the vesicular recycling and not the maturation of the MHC class II molecules themselves (Koppelman et al., 1997). It remains to be verified experimentally whether IL-10 would have any effect on the vesicular trafficking in order to modulate the MHC class I pathway. This question was not addressed in the present work. Several reports indicate that IL-10 may change expression levels of cathepsins in DCs (Fiebiger et al., 2001). We tested this hypothesis, but could not observe any effects on cathepsin expression/activity in B-cells expressing different forms of IL-10 (data not shown). Although the effects of IL-10 on the MHC class II pathway have been well studied, many aspects of the involvement of IL-10 remain to be determined.
Generation of antigen peptides for the MHC class I molecule is performed by several proteases in the cytosol. The 20S proteasome, which is one of the most studied proteases, does not seem to be affected by the presence of IL-10. There are also no obvious changes in non-proteasomal proteolytic activities by IL-10. This indicates that the changes in the peptide pool may not be affected by the presence of IL-10. However, our data suggests that IL-10 can possibly have a negative effect on immunoprotesome formation in the presence of INF-g. The counter-balance between different cytokines is a common theme and plays an important role in the modulation of the immune system. Our results are consistent with the notion that INF-g and IL-10 are counter players during the course of immune responses (Toungouz et al., 1996). It is well known that IL-10 shuts down the production of INF-g by macrophages. The balance between INF-g and IL-10 was shown to be shifted in different anti-angiogenic therapies, where a downregulation of Th1 cytokines, such as INF-g and an upregulation of IL-10 was observed (Gonzalez et al., 2000). The immunoproteasome, which is induced by INF-g (and TNF-a), is believed to generate a pool of peptides that are efficiently presented by the MHC class I molecules to the immune system (Sijts et al., 2000). Consequently, it is not surprising that IL-10 has a negative effect on the immunoproteasome formation. If this were the case, it would be the first indication of a cytokine-dependent negative regulation of immunoproteasome formation. However, to be able to confirm this hypothesis, additional experimental data will be required. For example, it has not yet been tested whether acute treatment of B cells with INF-g/IL-10 would lead to similar observations.
Due to IL-10’s anti-angiogenic properties (Cervenak et al., 2000), tumors expressing IL-10 can be exposed to hypoxic conditions. When cells were treated with CoCl2 to expose them to hypoxic conditions, we could not see any significant changes in proteasomal activities between the IL-10 expressing cells, and cells not expressing IL-10. However, we found a significant increase in non-proteasomal AAF-AMC hydrolysis in hIL-10 expressing cells. Primary sequence between the human and the viral form of IL-10 may explain the reasons why the viral form of IL-10 did not show any significant increased levels of enzymatic activity. Although there is an 84% homology between the proteins, they induce different physiological functions. hIL-10 has both immunosuppressive and immunostimulatory properties, while the viral form only has the immunosuppressive properties. Affinity of the human and viral form of IL-10 differs 1000-fold to the hIL-10 receptor (Ding et al., 2000). One way to explain the difference in AAF hydrolyzing activity could be the difference in affinity, which leads to different responses that may induce upregulation/modulation of cytosolic proteolysis. The shift in protease activity may be important for the generation of peptides to the antigenic pool presented on the surface of the cell. It is known that proteases such as Bleomycin hydrolase (BH) and puromycin-sensitive aminopeptidase (PSA) have a role in peptide trimming, a process normally downstream of the proteasome (Stoltze et al., 2000). An upregulation of these activities could lead to a more efficient peptide presentation, a scenario that would not favor viral pathogenisis. A shift of alternative pathways in protein proteolysis with c-myc translocation has been reported, in which TPPII is upregulated (Gavioli et al., 2001). Increase in TPPII activity is suggested to be involved in malignant tumors. These tumors are more resistant to inhibitors and can result in more metastases. The increase in AAF-AMC hydrolysis caused by hIL-10 in hypoxic treatment did not involve TPPII (fig.8), suggesting that other proteases such as BH and PSA could be involved. The involvement of these proteases was not tested, but may be something that should be addressed in future experiments. For many of these other proteases including BH and PSA, the role in the MHC class I antigen pathway, aside from peptide trimming, remains to be established.
Conclusions and future perspectives
IL-10 appears to have a negative effect on immunoproteasome formation in the presence of INF-g. If this were to be the case, it might represent the first evidence for a cytokine-dependent negative regulation of immunoproteasome formation. Furthermore, we may provide evidence that hIL-10 seems to have an effect on small peptidases with AFF-AMC hydrolyzing activity under hypoxic conditions.
The negative effect of immunoproteasome formation has to be further studied in more detail. The experiments have to be repeated several more times to be able to get more statistically based data. In addition, western blots with antibodies against the proteolytic subunits should be performed to be able to confirm the data using a different method, in addition to active site-directed probes. Treatment of wild type cells with exogenous IL-10 should also be performed in order to see if the effects can be induced and blocked, and also reversed with specific antibodies against IL-10. It will be interesting to study if there are differences between the chronic presence of IL-10 and the shorter exposure of the cells to the cytokine. Finally, cells could be exposed to different ratios of INF-g versus IL-10 prior to the analysis of components of cytosolic proteolysis. It would be interesting to extend this hypothesis to other APC’s, such as macrophages and DC’s.
The increased AAF-AMC hydrolyzing activity observed in hIL-10 expressing cells remains to be addressed in further detail. One of the objectives of further experimentation could be to determine the peptide pools generated under these conditions, and to identify the protease(s) with modulated activity and determine what role they might have in the MHC class I pathway.
5. Acknowledgments
I would like to thank Hidde Ploegh for giving me the opportunity to do my project in your laboratory.
I want to give Benedikt special thanks for being my guide and friend during this time. You have been inspirational to work with and a great person to learn from. I hope to be able to continue to do work together and giving me the opportunity to learn from a great scientist. I want to thank Claire-Lise, Celia and Collin for letting me borrow your husband/father from you.
A special thank to all members of the Ploegh lab for making this time fun and pleasant, and specially Dominic for helping me and being a great friend and guide during this time. Lucy for being a great technician and a sweet and kind person
I want to thank Maria-Teresa Bejarano for all the advice and help during my thesis.
I am thankful to my roommates at #8 Worthington Street, Aemro and Julio, for making the house lovely to live in and giving it a feeling of a small family. Special thanks to Warren for being a great friend and person, to discuss and do many small adventures with.
I want to give love to my family for having the understanding and support during my stay in Boston. I love you all and thank you for believing in me and giving me all your kindness and trust. I also want to give my love to my friends: Christofer, Nenad, Serhat and Ted for being more than just friend, but also persons who do the little extra that just special people like you guys do.
6. References
Arai, K. I., Lee, F., Miyajima, A., Miyatake, S., Arai, N., and Yokota, T. (1990). Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem 59, 783-836.
Balow, R. M., Tomkinson, B., Ragnarsson, U., and Zetterqvist, O. (1986). Purification, substrate specificity, and classification of tripeptidyl peptidase II. J Biol Chem 261, 2409-2417.
Baumeister, W., Walz, J., Zuhl, F., and Seemuller, E. (1998). The proteasome: paradigm of a self-compartmentalizing protease. Cell 92, 367-380.
Ben-Bassat, H., Goldblum, N., Mitrani, S., Goldblum, T., Yoffey, J. M., Cohen, M. M., Bentwich, Z., Ramot, B., Klein, E., and Klein, G. (1977). Establishment in continuous culture of a new type of lymphocyte from a "Burkitt like" malignant lymphoma (line D.G.-75). Int J Cancer 19, 27-33.
Bogyo, M., Shin, S., McMaster, J. S., and Ploegh, H. L. (1998). Substrate binding and sequence preference of the proteasome revealed by active-site-directed affinity probes. Chem Biol 5, 307-320.
Braun, B. C., Glickman, M., Kraft, R., Dahlmann, B., Kloetzel, P. M., Finley, D., and Schmidt, M. (1999). The base of the proteasome regulatory particle exhibits chaperone-like activity. Nat Cell Biol 1, 221-226.
Carmeliet, P., and Jain, R. K. (2000). Angiogenesis in cancer and other diseases. Nature 407, 249-257.
Cervenak, L., Morbidelli, L., Donati, D., Donnini, S., Kambayashi, T., Wilson, J. L., Axelson, H., Castanos-Velez, E., Ljunggren, H. G., Malefyt, R. D., et al. (2000). Abolished angiogenicity and tumorigenicity of Burkitt lymphoma by interleukin-10. Blood 96, 2568-2573.
Coux, O., Tanaka, K., and Goldberg, A. L. (1996). Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem 65, 801-847.
D'Andrea, A., Aste-Amezaga, M., Valiante, N. M., Ma, X., Kubin, M., and Trinchieri, G. (1993). Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med 178, 1041-1048.
de Waal Malefyt, R., Abrams, J., Bennett, B., Figdor, C. G., and de Vries, J. E. (1991). Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 174, 1209-1220.
Defrance, T., Vanbervliet, B., Briere, F., Durand, I., Rousset, F., and Banchereau, J. (1992). Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med 175, 671-682.
Deveraux, Q., Ustrell, V., Pickart, C., and Rechsteiner, M. (1994). A 26 S protease subunit that binds ubiquitin conjugates. J Biol Chem 269, 7059-7061.
Ding, Y., Qin, L., Kotenko, S. V., Pestka, S., and Bromberg, J. S. (2000). A single amino acid determines the immunostimulatory activity of interleukin 10. J Exp Med 191, 213-224.
Dubiel, W., Pratt, G., Ferrell, K., and Rechsteiner, M. (1992). Purification of an 11 S regulator of the multicatalytic protease. J Biol Chem 267, 22369-22377.
Fenteany, G., Standaert, R. F., Lane, W. S., Choi, S., Corey, E. J., and Schreiber, S. L. (1995). Inhibition of proteasome activities and subunit-specific amino-terminal threonine modification by lactacystin. Science 268, 726-731.
Fiebiger, E., Meraner, P., Weber, E., Fang, I. F., Stingl, G., Ploegh, H., and Maurer, D. (2001). Cytokines regulate proteolysis in major histocompatibility complex class II-dependent antigen presentation by dendritic cells. J Exp Med 193, 881-892.
Frentzel, S., Pesold-Hurt, B., Seelig, A., and Kloetzel, P. M. (1994). 20 S proteasomes are assembled via distinct precursor complexes. Processing of LMP2 and LMP7 proproteins takes place in 13-16 S preproteasome complexes. J Mol Biol 236, 975-981.
Gavioli, R., Frisan, T., Vertuani, S., Bornkamm, G. W., and Masucci, M. G. (2001). c-myc overexpression activates alternative pathways for intracellular proteolysis in lymphoma cells. Nat Cell Biol 3, 283-288.
Geier, E., Pfeifer, G., Wilm, M., Lucchiari-Hartz, M., Baumeister, W., Eichmann, K., and Niedermann, G. (1999). A giant protease with potential to substitute for some functions of the proteasome. Science 283, 978-981.
Glas, R., Bogyo, M., McMaster,
J. S., Gaczynska, M., and Ploegh, H. L. (1998). A proteolytic system that
compensates for loss of proteasome function. Nature 392, 618-622.
Glickman, M. H., Rubin, D. M., Fu, H., Larsen, C. N., Coux, O., Wefes, I., Pfeifer, G., Cjeka, Z., Vierstra, R., Baumeister, W., et al. (1999). Functional analysis of the proteasome regulatory particle. Mol Biol Rep 26, 21-28.
Gonzalez, S., Alcaraz, M. V., Cuevas, J., Perez, M., Jaen, P., Alvarez-Mon, M., and Villarrubia, V. G. (2000). An extract of the fern Polypodium leucotomos (Difur) modulates Th1/Th2 cytokines balance in vitro and appears to exhibit anti-angiogenic activities in vivo: pathogenic relationships and therapeutic implications. Anticancer Res 20, 1567-1575.
Griffin, T. A., Nandi, D., Cruz, M., Fehling, H. J., Kaer, L. V., Monaco, J. J., and Colbert, R. A. (1998). Immunoproteasome assembly: cooperative incorporation of interferon gamma (IFN-gamma)-inducible subunits. J Exp Med 187, 97-104.
Groettrup, M., Standera, S., Stohwasser, R., and Kloetzel, P. M. (1997). The subunits MECL-1 and LMP2 are mutually required for incorporation into the 20S proteasome. Proc Natl Acad Sci U S A 94, 8970-8975.
Groll, M., Ditzel, L., Lowe, J., Stock, D., Bochtler, M., Bartunik, H. D., and Huber, R. (1997). Structure of 20S proteasome from yeast at 2.4 A resolution. Nature 386, 463-471.
Jiang, B. H., Zheng, J. Z., Leung, S. W., Roe, R., and Semenza, G. L. (1997). Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem 272, 19253-19260.
Kessler, B. M., Tortorella, D., Altun, M., Kisselev, A. F., Fiebiger, E., Hekking, B. G., Ploegh, H. L., and Overkleeft, H. S. (2001). Extended peptide-based inhibitors efficiently target the proteasome and reveal overlapping specificities of the catalytic beta subunits. Chemistry & Biology 8, 913-929.
Kisselev, A. F., Akopian, T. N., Woo, K. M., and Goldberg, A. L. (1999). The sizes of peptides generated from protein by mammalian 26 and 20 S proteasomes. Implications for understanding the degradative mechanism and antigen presentation. J Biol Chem 274, 3363-3371.
Klein, G. (1983). Specific chromosomal translocations and the genesis of B-cell-derived tumors in mice and men. Cell 32, 311-315.
Knowlton, J. R., Johnston, S. C., Whitby, F. G., Realini, C., Zhang, Z., Rechsteiner, M., and Hill, C. P. (1997). Structure of the proteasome activator REGalpha (PA28alpha). Nature 390, 639-643.
Koppelman, B., Neefjes, J. J., de Vries, J. E., and de Waal Malefyt, R. (1997). Interleukin-10 down-regulates MHC class II alphabeta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity 7, 861-871.
Liu, Y., Wei, S. H., Ho, A. S., de Waal Malefyt, R., and Moore, K. W. (1994). Expression cloning and characterization of a human IL-10 receptor. J Immunol 152, 1821-1829.
Ljunggren, H. G., and Karre, K. (1990). In search of the 'missing self': MHC molecules and NK cell recognition. Immunol Today 11, 237-244.
Lowe, J., Stock, D., Jap, B., Zwickl, P., Baumeister, W., and Huber, R. (1995). Crystal structure of the 20S proteasome from the archaeon T. acidophilum at 3.4 A resolution. Science 268, 533-539.
Ma, C. P., Slaughter, C. A., and DeMartino, G. N. (1992). Identification, purification, and characterization of a protein activator (PA28) of the 20 S proteasome (macropain). J Biol Chem 267, 10515-10523.
Matsuda, M., Salazar, F., Petersson, M., Masucci, G., Hansson, J., Pisa, P., Zhang, Q. J., Masucci, M. G., and Kiessling, R. (1994). Interleukin 10 pretreatment protects target cells from tumor- and allo-specific cytotoxic T cells and downregulates HLA class I expression. J Exp Med 180, 2371-2376.
Matthes, T., Werner-Favre, C., Tang, H., Zhang, X., Kindler, V., and Zubler, R. H. (1993). Cytokine mRNA expression during an in vitro response of human B lymphocytes: kinetics of B cell tumor necrosis factor alpha, interleukin (IL)6, IL-10, and transforming growth factor beta 1 mRNAs. J Exp Med 178, 521-528.
Moore, K. W., O'Garra, A., de Waal Malefyt, R., Vieira, P., and Mosmann, T. R. (1993). Interleukin-10. Annu Rev Immunol 11, 165-190.
Morel, A. S., Quaratino, S.,
Douek, D. C., and Londei, M. (1997). Split activity of interleukin-10 on
antigen capture and antigen presentation by human dendritic cells: definition
of a maturative step. Eur J Immunol 27, 26-34.
Mosmann, T. R. (1991). Role of a new cytokine, interleukin-10, in the cross-regulation of T helper cells. Ann N Y Acad Sci 628, 337-344.
Mosmann, T. R., and Moore, K. W. (1991). The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today 12, A49-53.
Parham, P., Barnstable, C. J., and Bodmer, W. F. (1979). Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol 123, 342-349.
Preckel, T., Fung-Leung, W. P., Cai, Z., Vitiello, A., Salter-Cid, L., Winqvist, O., Wolfe, T. G., Von Herrath, M., Angulo, A., Ghazal, P., et al. (1999). Impaired immunoproteasome assembly and immune responses in PA28-/- mice. Science 286, 2162-2165.
Rehm, A., Stern, P., Ploegh, H. L., and Tortorella, D. (2001). Signal peptide cleavage of a type I membrane protein, HCMV US11, is dependent on its membrane anchor. Embo J 20, 1573-1582.
Rousset, F., Garcia, E., Defrance, T., Peronne, C., Vezzio, N., Hsu, D. H., Kastelein, R., Moore, K. W., and Banchereau, J. (1992). Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A 89, 1890-1893.
Salazar-Onfray, F. (1999). Interleukin-10: a cytokine used by tumors to escape immunosurveillance. Med Oncol 16, 86-94.
Salazar-Onfray, F., Charo, J., Petersson, M., Freland, S., Noffz, G., Qin, Z., Blankenstein, T., Ljunggren, H. G., and Kiessling, R. (1997). Down-regulation of the expression and function of the transporter associated with antigen processing in murine tumor cell lines expressing IL-10. J Immunol 159, 3195-3202.
Salazar-Onfray, F., Petersson, M., Franksson, L., Matsuda, M., Blankenstein, T., Karre, K., and Kiessling, R. (1995). IL-10 converts mouse lymphoma cells to a CTL-resistant, NK-sensitive phenotype with low but peptide-inducible MHC class I expression. J Immunol 154, 6291-6298.
Schmidt, M., and Kloetzel, P. M. (1997). Biogenesis of eukaryotic 20S proteasomes: the complex maturation pathway of a complex enzyme. Faseb J 11, 1235-1243.
Schmidtke, G., Eggers, M., Ruppert, T., Groettrup, M., Koszinowski, U. H., and Kloetzel, P. M. (1998). Inactivation of a defined active site in the mouse 20S proteasome complex enhances major histocompatibility complex class I antigen presentation of a murine cytomegalovirus protein. J Exp Med 187, 1641-1646.
Seder, R. A., Gazzinelli, R., Sher, A., and Paul, W. E. (1993). Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon gamma production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci U S A 90, 10188-10192.
Sijts, A. J., Ruppert, T., Rehermann, B., Schmidt, M., Koszinowski, U., and Kloetzel, P. M. (2000). Efficient generation of a hepatitis B virus cytotoxic T lymphocyte epitope requires the structural features of immunoproteasomes. J Exp Med 191, 503-514.
Stoltze, L., Schirle, M., Schwarz, G., Schroter, C., Thompson, M. W., Hersh, L. B., Kalbacher, H., Stevanovic, S., Rammensee, H. G., and Schild, H. (2000). Two new proteases in the MHC class I processing pathway. Nat Immunol 1, 413-418.
Strickland, E., Hakala, K., Thomas, P. J., and DeMartino, G. N. (2000). Recognition of misfolding proteins by PA700, the regulatory subcomplex of the 26 S proteasome. J Biol Chem 275, 5565-5572.
Terrazzano, G., Romano, M. F., Turco, M. C., Salzano, S., Ottaiano, A., Venuta, S., Fontana, S., Manzo, C., Zappacosta, S., and Carbone, E. (2000). HLA class I antigen downregulation by interleukin (IL)-10 is predominantly governed by NK-kappaB in the short term and by TAP1+2 in the long term. Tissue Antigens 55, 326-332.
Thompson-Snipes, L., Dhar, V., Bond, M. W., Mosmann, T. R., Moore, K. W., and Rennick, D. M. (1991). Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J Exp Med 173, 507-510.
Tortorella, D., Story, C. M., Huppa, J. B., Wiertz, E. J., Jones, T. R., Bacik, I., Bennink, J. R., Yewdell, J. W., and Ploegh, H. L. (1998). Dislocation of type I membrane proteins from the ER to the cytosol is sensitive to changes in redox potential. J Cell Biol 142, 365-376.
Toungouz, M., Denys, C., de Groote, D., Andrien, M., and Dupont, E. (1996). Optimal control of interferon-gamma and tumor necrosis factor-alpha by interleukin-10 produced in response to one HLA-DR mismatch during the primary mixed lymphocyte reaction. Transplantation 61, 497-502.
Wang, E. W., Kessler, B. M., Borodovsky, A., Cravatt, B. F., Bogyo, M., Ploegh, H. L., and Glas, R. (2000). Integration of the ubiquitin-proteasome pathway with a cytosolic oligopeptidase activity. Proc Natl Acad Sci U S A 97, 9990-9995.
Wang, Z. Q., Bapat, A. S., Rayanade, R. J., Dagtas, A. S., and Hoffmann, M. K. (2001). Interleukin-10 induces macrophage apoptosis and expression of CD16 (FcgammaRIII) whose engagement blocks the cell death programme and facilitates differentiation. Immunology 102, 331-337.
Vieira, P., de Waal-Malefyt, R., Dang, M. N., Johnson, K. E., Kastelein, R., Fiorentino, D. F., deVries, J. E., Roncarolo, M. G., Mosmann, T. R., and Moore, K. W. (1991). Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci U S A 88, 1172-1176.
Voges, D., Zwickl, P., and Baumeister, W. (1999). The 26S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem 68, 1015-1068.
Yssel, H., De Waal Malefyt, R., Roncarolo, M. G., Abrams, J. S., Lahesmaa, R., Spits, H., and de Vries, J. E. (1992). IL-10 is produced by subsets of human CD4+ T cell clones and peripheral blood T cells. J Immunol 149, 2378-2384.

