image gallery
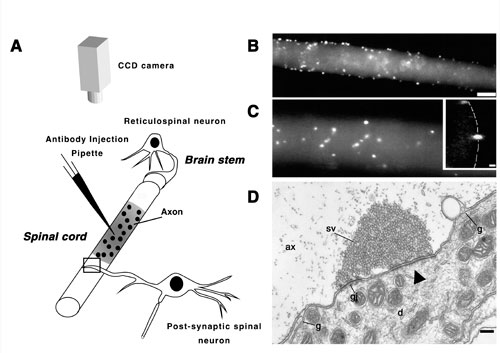
Morphological organization of the reticulospinal synapse. A: Schematic diagram showing the structural relation between a reticulospinal neuron and a spinal neuron in lamprey, and the experimental paradigm used to visualize active zones in the living giant axon (shown in B and C). An antiserum against the synaptic vesicle-associated protein synapsin was labeled with Cy5 and pressure-injected into the axon. The distribution of fluorescently tagged antibodies was monitored with a CCD detector. Injection of the antibodies into lamprey reticulospinal axons resulted in accumulation of fluorescence in spots, which are shown in B and C at different magnifications. The spots indicate the position of active zones (see also Pieribone et al., 1995). Bar in B, 25 um for B and 15 um for C. Inset in C is a cross-sectional confocal image of an injected axon, which reveals that spots are localized to the inner surface of the axonal membrane (stippled line). Bar for the inset 2 um. D: Electron micrograph of a reticulospinal synapse in a region of the axon outside the site of injection (indicated by a rectangle in A). Designations: sv-synaptic vesicle cluster; ax- axoplasmic matrix; gj-gap junction; g-glia; d- dendrite of a postsynaptic cell; arrowhead indicates the active zone. Bar, 0.2 um. Note the presence of specializations of both electrical and a chemical synapse in the same intracellular contact.
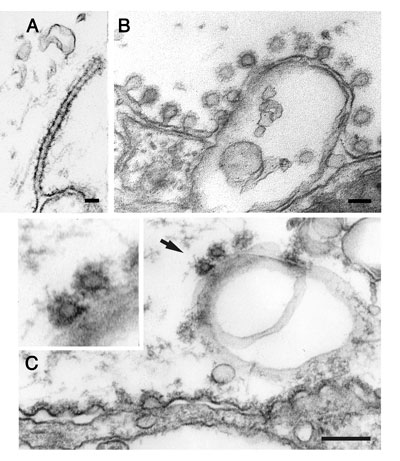
Perturbation of endocytosis by microinjections. (a) Clathrin coated pit with a long tubular neck surrounded by electron-dense rings, which was induced by presynaptic microinjection of GTPyS. (b) Invaginated clathrin-coated pits at the plasma membrane adjacent to an active zone in a giant reticulospinal axon. The axon had been injected with a GST fusion protein containing the SH3 domain of amphiphysin and subjected to action potential stimulation. (c) Invaginated clathrin coated pits at endosome-like plasma membrane invaginations in a synaptic region of a reticulospinal axon. The axon had been injected with endophilin antibodies. Note that shallow coated pits occur at the plasma membrane adjacent to the active zone (located just to the left of the image field), while invaginated coated pits are only seen at the invaginated part of the plasma membrane.
Recent results: Intersectin in synaptic vesicle recycling
Emma Evergren, Helge Gad, Kristin Walther, Anna Sundborger, Nikolay Tomilin
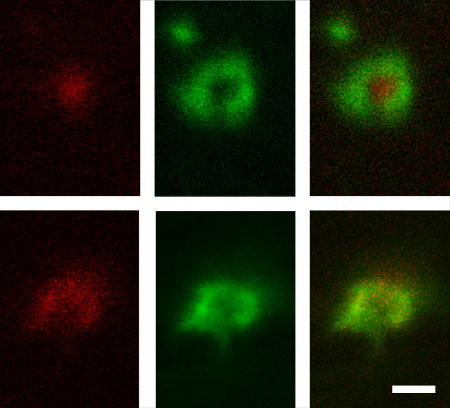
Localization of intersectin in living lamprey reticulospinal axons. Confocal images of reticulospinal synapses double injected with LIS-AC antibodies (red) and phalloidin (green). Images were acquired in axons at rest and after 20 min stimulation at 5 Hz.
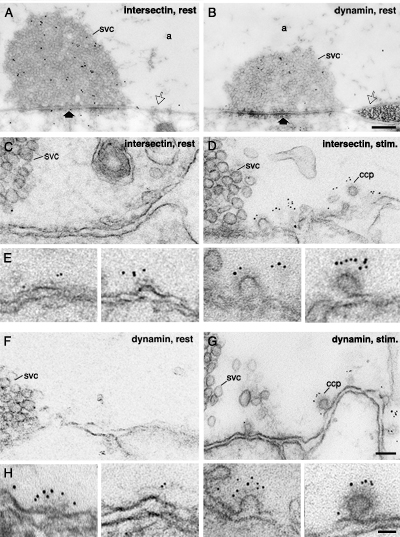
Redistribution of intersectin immunoreactivity in the giant reticulospinal synapse in response to stimulation.(A, B) Electron micrographs of reticulospinal synapses fixed at rest and labeled with LIS-AC (A) and dynamin (B) antibodies using post-embedding immunogold technique. The thick arrow marks the active zone. Open arrow indicates the periactive zone. (C, D and F, G) Periactive zones of reticulospinal synapses fixed at rest (C, F) and during K+-stimulation (D, G) labeled with the LIS-AC (C, D) and dynamin (F, G) antibodies using a pre-embedding immunogold protocol. Note an increase in the number of gold particles in the periactive zones of stimulated compared to resting synapses. (E, H) Electron micrographs showing association of intersectin (E) and dynamin (H) immunoreactivity with clathrin-coated intermediates at different stages. a: axoplasmic matrix; svc: synaptic vesicle cluster; ccp: clathrin-coated pit. Scale bars, A, B: 0.4 um; C, D, F, G: 0.1 um; E, H: 0.05 um.
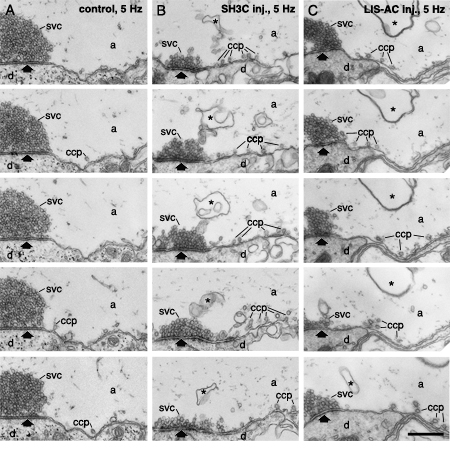
Serial section analysis of synapses microinjected with GST-SH3C and LIS-AC antibodies. (A) Electron micrographs of five ultrathin sections from an uninjected reticulospinal synapse stimulated at 5 Hz. The synapse was cut in serial sections. Images show a part of this series. Every other section is shown. (B) Electron micrographs of five ultrathin sections from a synapse microinjected with GST-SH3C domain, analyzed as in A. (C) Electron micrographs of five ultrathin sections from a synapse microinjected with LIS-AC antibodies, analyzed as in A. Designations as in Figs. 3, 4. Scale bar 0.4 um.
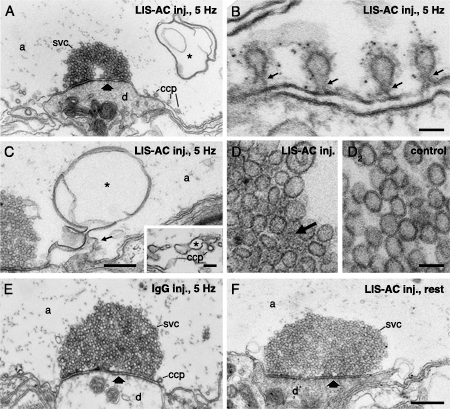
Effects of acute perturbation of intersectin SH3 domain interactions by antibodies. (A-D) Electron micrographs of synapses in reticulospinal axons stimulated at 5 Hz for 30 min after microinjection of LIS-AC antibodies. Note the accumulation of constricted coated pits (A, B) and membrane infolds (asterisk; A, C) at the periactive zone. Constricted coated pits in B were in addition labeled with dynamin antibodies (LD-1). Thin arrows point to constricted collared necks. (C) Electron micrograph of a membrane infold connected to the plasma membrane (thin arrow) as revealed by serial section analysis. Inset shows a membrane infold with a budding clathrin-coated pit. (D) Electron micrographs showing synaptic vesicle clusters from an axon microinjected with LIS-AC (1) and an uninjected control axon (2). Note a dense cytomatrix between synaptic vesicles in D1 (arrow). (E) A synapse from an axon microinjected with unspecific IgG and stimulated at 5 Hz. (F) Electron micrograph of a resting reticulospinal synapse after LIS-AC antibody microinjection. The average number of synaptic vesicles in microinjected synapses were not significantly different from uninjected resting synapses (p>0.05; two-tailed Student’s t-test, n=14). Scale bars, A, E, F: 0.5 um; B: 0.05 um; C: 0.3 um; D: 0.05 um; inset in C: 0.1 um.
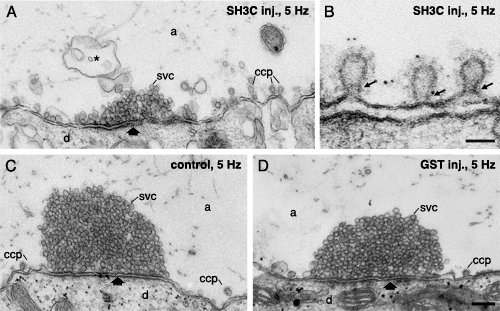
Microinjection of intersectin GST-SH3C domain inhibits synaptic vesicle recycling. (A, B) Electron micrographs of reticulospinal synapses microinjected with the GST-SH3C domain of intersectin and stimulated at 5 Hz for 30 min. Note the reduction of vesicles clustered at active zones and an accumulation of clathrin-coated pits and membrane invaginations (asterisk) compared to an uninjected synapse (C). (B) Electron micrograph showing constricted collared clathrin-coated pits. Thin arrows point to constricted necks with collars. In this experiment the synapse was in addition labeled with amphiphysin antibodies using the immunogold technique. (C) Electron micrograph of an uninjected control synapse stimulated at 5 Hz. (D) Electron micrograph showing a synapse microinjected with GST. Scale bars, A, C, D: 0.2 um; B: 0.05 um.
Perturbation of intersectin-dynamin interactions results in changes in dynamin distribution at clathrin-coated pits. (A-C) Low power electron micrographs of periactive zones labeled with dynamin antibodies from a control synapse (A), GST-SH3C injected synapse (B), and a synapse where LIS-AC antibodies were microinjected (C). Scale bar: A-C: 0.2 um.
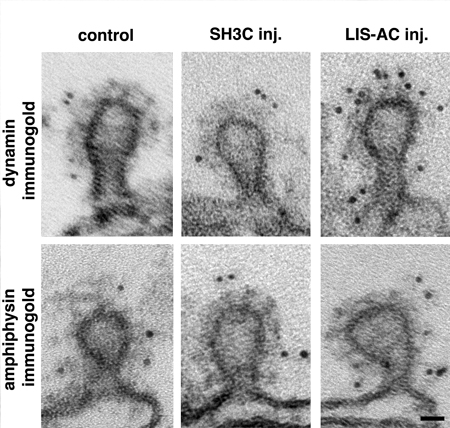
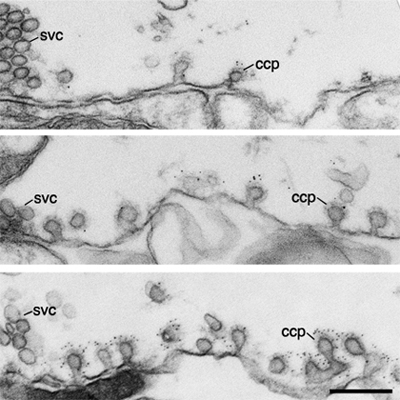
Perturbation of intersectin-dynamin interactions results in changes in dynamin distribution at clathrin-coated pits. Electron micrographs of coated intermediates from control synapses (far away from the microinjection site; first column) and from synapses where GST-SH3C domain (second column) and LIS-AC antibodies (third column) were microinjected. Coated pits were immunolabeled using dynamin (first row) and amphiphysin (second row) antibodies. Note the increase in dynamin labeling on coated pits after LIS-AC microinjection compared to control. Scale bar: 0.02 um.
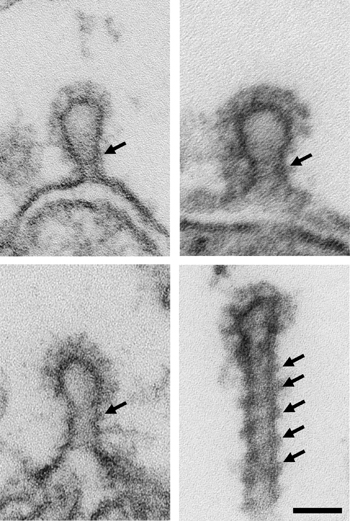
High-power micrographs showing deeply invaginated coated pits with electron dense collars (arrows) from periactive zones of stimulated synapses (5 Hz, 30 min) microinjected with GST-SH3C, LIS-AC antibodies, non-injected control, and GTPyS. Note the difference in appearance of the collars in synapses microinjected with intersectin reagents compared to control and GTPyS. Scale bar: 0.05 um.
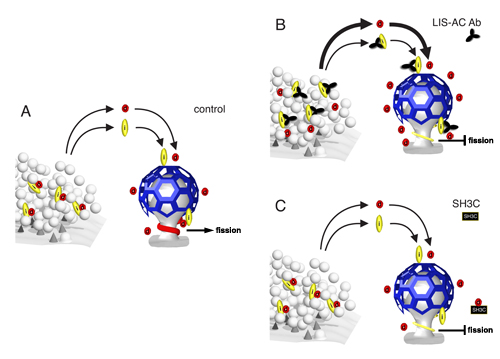
Intersectin functions as a negative regulator of dynamin recruitment to clathrin-coated pits and directs dynamin to the fission complex. (A) Intersectin (i) and dynamin (d) are localized to the synaptic vesicle cluster and redistribute to sites of endocytosis during activity, where they associate with clathrin-coated pits. Dynamin, and other components of the fission complex, assemble in a collar around the neck of the pit and sever the membrane. (B) Intersectin antibodies perturb interactions with dynamin, leading to an increase in dynamin redistribution to endocytic zones. We hypothesize that intersectin acts as a negative regulator of dynamin recruitment to endocytic zones. Furthermore, blocking dynamin-intersectin interactions either by antibodies or with the GST-SH3C domain resulted in formation of thinner fission complexes and an inhibition of membrane scission (B, C), thus suggesting that intersectin actively directs dynamin to the fission complex. As shown in synapses microinjected with the LIS-AC antibody (B), it is not sufficient to have high amounts of dynamin at the membrane neck of clathrin-coated pits to achieve efficient fission; an active recruitment of dynamin to the neck seems to be required.










